Copyright 2012 neutronsources.org | All rights reserved. | Powered by FRM II | Imprint / Privacy Policy
Quenched microemulsions: a new route to proton conductors
By A. Menelle, 07/08/2014
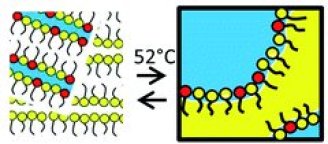
Solid-state proton conductors operating under mild temperature conditions (T < 150 °C) would promote the use of electrochemical devices as fuel cells. Alternatives to the water-sensitive membranes made of perfluorinated sulfonated polymers require the use of protogenic moieties bearing phosphates/phosphonates or imidazole groups. Here, we formulate microemulsions using water, a cationic surfactant (cetyltrimethyl ammonium bromide, CTAB) and a fatty acid (myristic acid, MA). The fatty acid acts both as an oil phase above its melting point (52 °C) and as a protogenic moiety. We demonstrate that the mixed MA–CTA film presents significant proton conductivity. Furthermore, bicontinuous microemulsions are found in the water–CTAB–MA phase diagram above 52 °C, where molten MA plays both the role of the oil phase and the co-surfactant. This indicates that the hydrogen-bond rich MA–CTA film can be formulated in the molten phase. The microemulsion converts into a lamellar phase upon solidification at room temperature. Our results demonstrate the potential of such self-assembled materials for the design of bulk proton conductors, but also highlight the necessity to control the evolution of the nanostructure upon solidification of the oil phase.
Original Publication:
Quenched microemulsions: a new route to proton conductors
C. Noirjean, F. Testard, J. Jestin, O. Taché, C. Dejugnat and D. Carriere
DOI: 10.1039/C4SM00849A